IRG-III: A Fundamentally New Approach to Fuel Cells
A society powered by renewable energy sources will necessarily rely on a wide array of electrochemical processes and systems. Since sustainability requires high efficiency, electrochemical devices will inevitably be an integral part of our energy future. Here we will study fuel cells from a broader and more fundamental paradigm than is typical. As a cornerstone of this effort we are interested in ionic liquid electrolytes. This relatively new class of electrolytes enables the study of new electrocatalysts and fuel cell architectures, and also opportunities for fundamental science. This paradigm may provide a host of potentially game changing-transformative outcomes for fuel cells.
There are a series of fundamental issues associated with all electrochemical systems. One is that the conductivity of all electrolytes has an upper limit, the conductivity which is fixed by the root mean square velocity of vibrating ions. If a breakthrough in conductivity making the IR limit accessible, could be achieved, the efficiency of fuel cells could be significantly improved. Also, in any electrochemical cell the various components (electrolyte, electrodes, redox couple) may degrade over time. In the case of a proton exchange membrane (PEM) fuel cell, the degradation of the membrane and the catalyst severely limit the longevity of the cell. Another issue is the overpotential required to drive a reaction at an appreciable rate. A large overpotential for oxygen reduction in acid fuel cells is the largest single contributor to efficiency loss. Thus finding higher activity and non-precious metal catalysts is of primary importance. Further (especially important in the context of fuel cells), the combination of cell geometry and required components has a dominant impact on all of the above issues. For example, the membrane in a PEM dictates, among other parameters, operating temperature, humidity, catalyst selection, and fuel selection. A fuel cell architecture that can eliminate the membrane and the associated constraints would have a significant impact on performance, operating conditions, and component selection.
 This section outlines a well directed path for making major, potentially transformative advances in the central issues of IR losses, catalytic activity, catalyst stability, and fundamentally new fuel cell architectures. The focus of this effort is purposely different from the narrow region of parameter space defined by PEM fuel cells, Nafion membranes, and Pt catalysts. That technology is well established; there is little likelihood that refining it will produce game-changing results. The present work steps out of this (heavily funded) approach. This section outlines a well directed path for making major, potentially transformative advances in the central issues of IR losses, catalytic activity, catalyst stability, and fundamentally new fuel cell architectures. The focus of this effort is purposely different from the narrow region of parameter space defined by PEM fuel cells, Nafion membranes, and Pt catalysts. That technology is well established; there is little likelihood that refining it will produce game-changing results. The present work steps out of this (heavily funded) approach.
Ionic Liquids
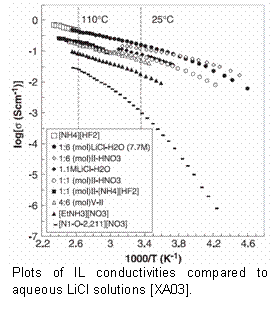
Ionic liquids (ILs) are liquid salts that have melting points typically below 100 ºC. ILs can have conductivities that currently rival those of aqueous electrolytes. The electrochemical stability range of ILs is exceptional, reaching 6 V in some cases [MYT+00]. It was realized only three years ago that protic ionic liquids could serve as fuel cell electrolytes. ASU might have the first patent on this technology. We showed that, at low loads, theoretical energy conversion efficiencies could be obtained for some cases, perhaps due to the tunability of the proton activity. Even more recently we reported the first use of inorganic pILs for fuel cells. The pIL electrolytes are neither acidic nor basic, but lie tunably in between. This opens the door to the use of novel inexpensive catalysts. To realize this potential, fundamental electrochemical studies are needed.
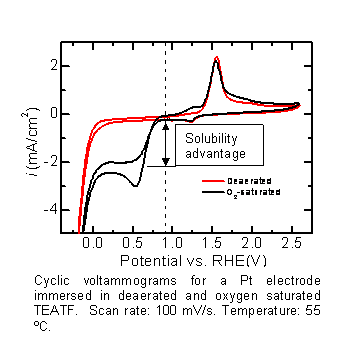 As a first step we are examining the electrochemical stability of four high conductivity pILs: triethylammonium triflate (TEATF), dimethylethylammonium triflate (DMEATF), ethylammonium nitrate (EAN), and triethylammonium methane sulfonate (TEAMS). We have also examined the overpotential for oxygen reduction and hydrogen oxidation in each of these systems. Oxygen reduction in TEATF on a Pt electrode has the turn-on potential at 910 mV vs. RHE (reversible hydrogen electrode), almost as positive as the typical 950 mV observed in aqueous systems. Another important observation is that the solubility of oxygen in these electrolytes is at least an order of magnitude larger than that for aqueous electrolytes, resulting in diffusion limited current densities of >2 mA/cm2. This higher solubility along with other factors could lead to altogether new fuel cell architectures, as discussed in the Architectures section below. As a first step we are examining the electrochemical stability of four high conductivity pILs: triethylammonium triflate (TEATF), dimethylethylammonium triflate (DMEATF), ethylammonium nitrate (EAN), and triethylammonium methane sulfonate (TEAMS). We have also examined the overpotential for oxygen reduction and hydrogen oxidation in each of these systems. Oxygen reduction in TEATF on a Pt electrode has the turn-on potential at 910 mV vs. RHE (reversible hydrogen electrode), almost as positive as the typical 950 mV observed in aqueous systems. Another important observation is that the solubility of oxygen in these electrolytes is at least an order of magnitude larger than that for aqueous electrolytes, resulting in diffusion limited current densities of >2 mA/cm2. This higher solubility along with other factors could lead to altogether new fuel cell architectures, as discussed in the Architectures section below.
Theory
In electrochemical systems the details of the electrode/electrolyte interface determine such characteristics as the overpotential to produce a particular reaction. In aqueous environments the interphase region is reasonably well understood, yet there are a number of unanswered questions even there. Also, the activity of a particular electrocatalyst for oxygen reduction in aqueous environments has long been known to strongly correlate to a low OH adsorption energy. Does this hold true in pILs?
Understanding the exact details of the structure of the interphase, and e.g. adsorption processes of species present in pILs, so as to guide catalyst and IL selection, is a key fundamental component of this work. The structure of the IL-metal interface is a result of the electronic structure of the system. Modeling of this interface through accurate electronic structure methods can shed a great deal of light on what is occurring and the dominant factors that lead to a particular structure. Analysis of these surface phenomena within the context of ab initio methods poses a significant challenge. Next-generation ab initio tools based on the LDA and GW approximations will be developed in the course of this project, to make it possible to realistically model these effects.
Architecture
We are currently exploring two membraneless fuel cell architectures, both of which should capitalize on the characteristics of pILs. One of the architectures is in the provisional patent stage and is capable of generating in excess of 50 mW/cm2 in aqueous systems. The other architecture will be a mixed fuel-air fuel cell that relies on selective catalysts for operation. Our preliminary work indicates that there may exist higher oxidation-reduction selectivities for certain catalysts in pILs as compared to aqueous systems. Additionally, the apparent order-of-magnitude increase in O2 and H2 saturation limit for dissolved gases may also enable an all liquid fuel cell. This type of fuel cell is precluded in aqueous systems due to the low saturation limit of dissolved gaseous reactants. In sum, a fundamentally new electrochemical environment should lead to new fuel cell architectures that capitalize on the properties of pILs and pIL-optimized electrocatalysts.
|